Introduction
After cardiac injury from ventricular ablation or myocardial infarction, scar tissue is formed by non-myocytes (NM) and extracellular matrix, replacing apoptotic and necrotic cells. In scar tissue, especially in the border zone, NM can electrotonically couple to neighbouring cardiomyocytes (CM) via connexin-based gap junctions1,2. In this study, we aimed to characterize the 3D structure and electrophysiological role of fibroblasts (FB) and macrophages (MΦ) in cryo-ablated mouse hearts. FB and MΦ were selectively labelled by a light-gated cation channel, channelrhodopsin-2 (ChR2), linked to enhanced yellow fluorescent protein (eYFP). While membrane-localized eYFP served as marker for 3D structural reconstructions of FB, optogenetic depolarization of NM was used to assess their specific effects on left-ventricular (LV) electrophysiology.
Methods
We used Tcf21-MerCreMer and Cx3cr1-CreERT2 mice to target ChR2-eYFP to FB and MΦ, respectively3, and littermates not expressing ChR2 as negative controls for optogenetic experiments. Langendorff-perfused hearts were optically stimulated and electrically paced to probe the effects of ChR2-mediated FB or MΦ depolarization on action potential (AP) shape, restitution, and conduction velocity, assessed by dye-based optical mapping. We compared the scar, border zone and remote LV of cryo-ablated hearts to sham-operated hearts, harvested 28 days after surgery. To structurally characterize fluorescently labelled NM, we optically cleared hearts using an adapted X-CLARITY protocol and imaged them with confocal microscopy4,5. We generated 3D models of FB and MΦ, and quantitatively assessed their morphology, distribution, surface area and fractional volume in healthy, sham-operated and cryo-ablated hearts.
Results and Conclusions
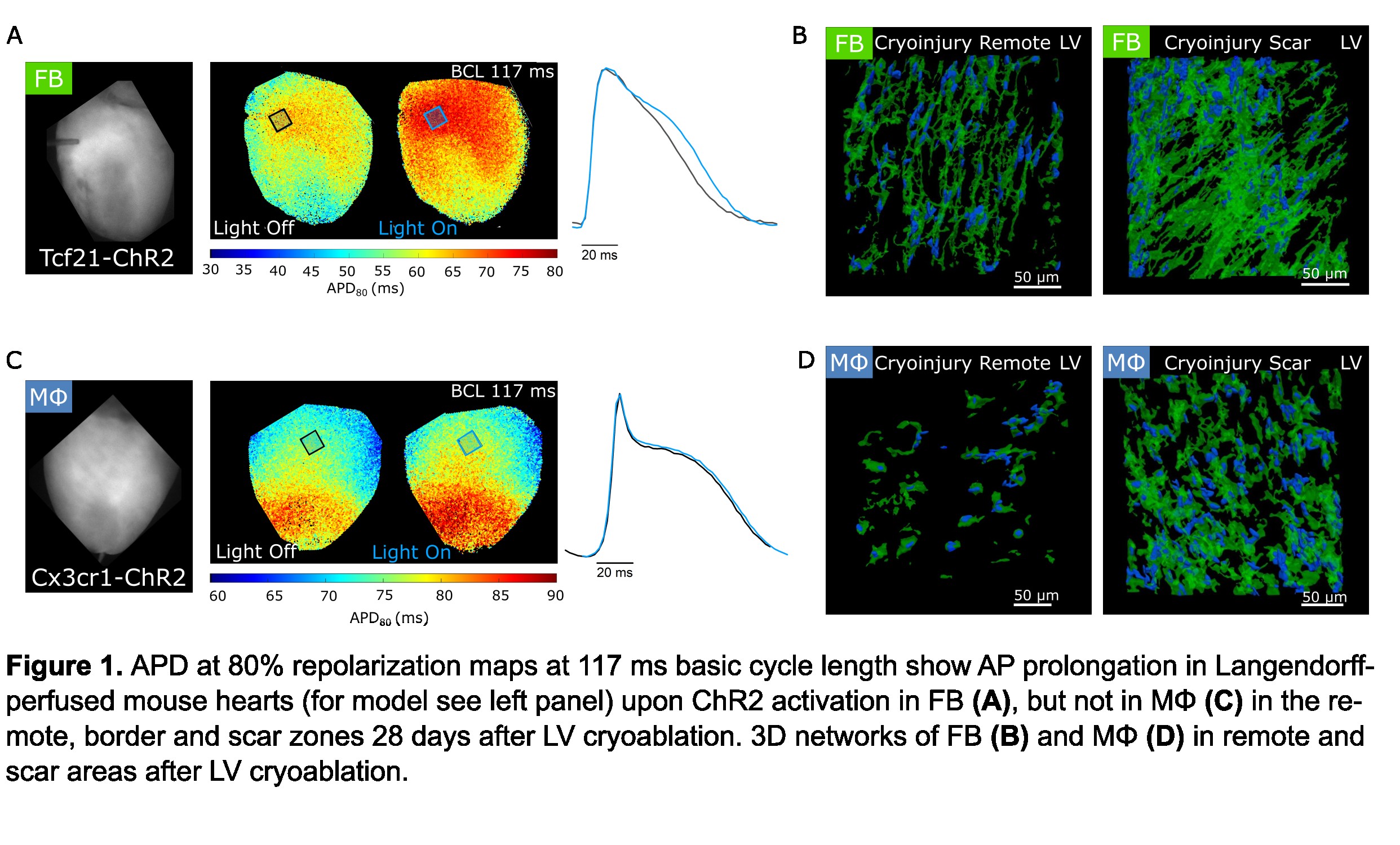
The main findings are: (i) FB depolarization by ChR2 activation in cryo-ablated hearts prolong CM APD in scar, border zone, and remote tissue. (ii) Illumination of sham-operated that expressed ChR2 and cryo-ablated hearts not expressing ChR2 did not show any effect on APD. (iii) In contrast to FB, ChR2-mediated depolarization of MФ did not affect ventricular APD in cryo-ablated hearts. Our structural data shows that: (iv) FB in healthy hearts have elongated shapes and thin branches, forming interconnected networks that follow the orientation of CM. (v) MΦ morphology is not dissimilar to FB, but they are arranged as solitary cells. (vi) In cryo-ablated hearts, both FB and MΦ are abundant in the scar and border zone, whereas only FB numbers are increased in remote LV myocardium. Accordingly, the volume fraction occupied by FB and MΦ in healthy ventricles were 2.5±0.3%, n = 11 and 1.6±0.2%, n = 8 (mean±SEM, n = analyzed regions). In cryo-ablated hearts, FB and MΦ occupy a volume fraction of 13.0±0.9%, n= 15 and 7.6±0.5%, n = 10 in the scar, and 7.8±0.3%, n = 16 and 2.0±0.2%, n = 10 in remote tissue from the same hearts. Our study highlights the electrophysiological relevance of FB, in particular in lesioned tissue, and reveals key structural characteristics of FB and MΦ in situ. Future work will focus on the role of FB in arrhythmogenesis.
References
[1] Quinn T A. et al. Proc Natl Acad. Sci.2016/113:14852–14857
[2] Wang Y. et al. Science. 2023/381:1480–1487
[3] Fernández MC et al. Methods Mol Biol. 2021/2191:287–307
[4] Simon-Chica A. et al. Cardiovascular Res. 2022/118:798–813
[5] Fernández M C. et al. BioRxiv. 2023/ https://doi.org/10.1101/2023.11.30.569388
