Background and aim: In clinical practice, women are often more likely than men to be underdiagnosed with, and undertreated for, cardiovascular (CV) disease, and are underrepresented in most lipid-lowering therapy (LLT) trials. Additional real-world data are needed to understand the reasons for these differences and thus optimise CV risk reduction in both women and men. This post-hoc analysis of the German cohort of the MILOS study (NCT04579367), which included a higher % of women than usually observed in LLT trials, evaluated differences between men and women in LLT use and low-density lipoprotein cholesterol (LDL-C) levels during the 2 years’ follow-up.
Methods: The MILOS study is an ongoing, European, prospective, non-interventional study evaluating the effectiveness and safety of bempedoic acid (BA) or BA + ezetimibe (EZE) fixed-dose combination (FDC) in a real-world setting. Adult patients with hypercholesterolaemia or mixed dyslipidaemia were recruited from January 2021 to January 2022. LLT use, change in LDL-C levels and the proportion of patients achieving LDL-C goals were compared between women and men prior to initiation of BA or BA+EZE FDC and at 2 years’ follow-up. Adverse drug reactions (ADRs) through 2 years’ follow-up were also compared.
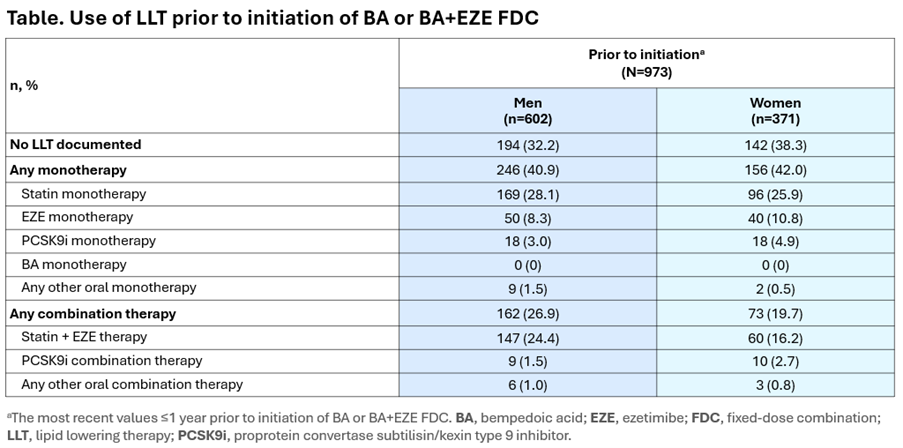
Results: Baseline data from 973 patients (371 [38.1%] women) were analysed. At baseline, women were slightly older (mean [standard deviation] [SD]) age (66.7 [9.4] years) than men (63.7 [10.2] years). Overall, 8.1% of women were considered low/moderate CV risk, 34.2% high CV risk and 57.7% very high CV risk, vs 2.7%, 24.8% and 72.6% of men, respectively. Prior to initiation of BA or BA+EZE FDC, women were less likely than men to be receiving LLT. Among patients on LLT, women were less likely than men to be receiving combination therapy, with similar proportions receiving monotherapy (Table).
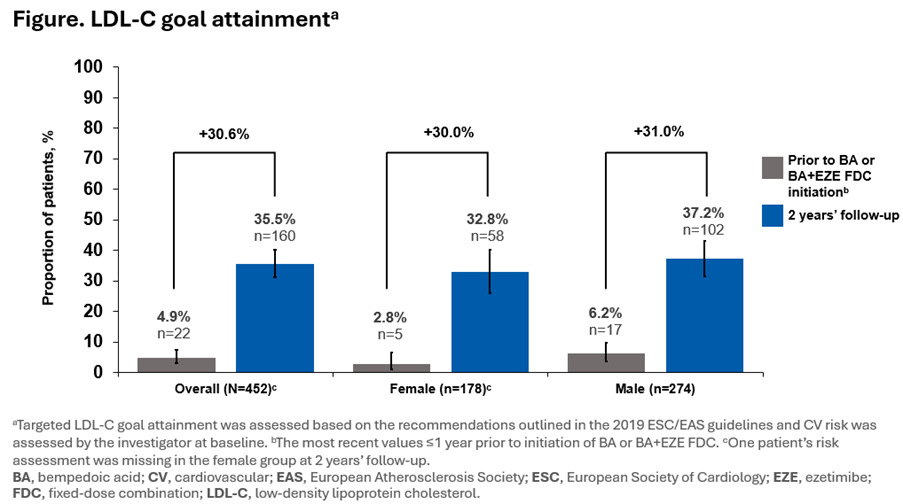
Overall, 452 patients (178 [39.4%] women) had LDL-C data available at each timepoint. Prior to initiation of BA or BA+EZE FDC, mean (SD) LDL-C levels were higher in women (136.60 [51.22] mg/dL) than in men (111.42 [43.76] mg/dL). At 2 years’ follow-up, mean (SD) LDL-C levels remained higher in women (84.16 [37.55] mg/dL) than in men (72.59 [31.92] mg/dL); however, the mean (SD) relative reduction at 2 years’ follow-up was slightly higher in women (33.0% [31.8]) than in men (28.6% [38.2]). The proportion of patients at LDL-C goal increased similarly (~30%) in both sexes (Figure). Over the 2 years’ follow-up, ADRs related to BA or BA+EZE FDC occurred in 14.3% (n=53) of women and in 9.0% (n=54) of men. No new safety signals were observed during this analysis vs the CLEAR clinical trial programme.
Conclusion: In the German cohort of the MILOS study, women had higher mean LDL-C levels and were less likely to be receiving LLT vs men prior to initiation of BA or BA+EZE FDC. At 2 years’ follow-up, more than 80% of women and men were receiving BA in combination with other LLTs. The addition of BA, with or without other LLTs, was associated with a reduction in LDL-C levels in both sexes. Furthermore, a comparable increase in the proportion of patients achieving their LDL-C goals was observed across both sexes over 2 years’ follow-up. These findings highlight the importance of the use of combination LLTs to address disparities in LDL-C levels between women and men and to improve CV outcomes.
