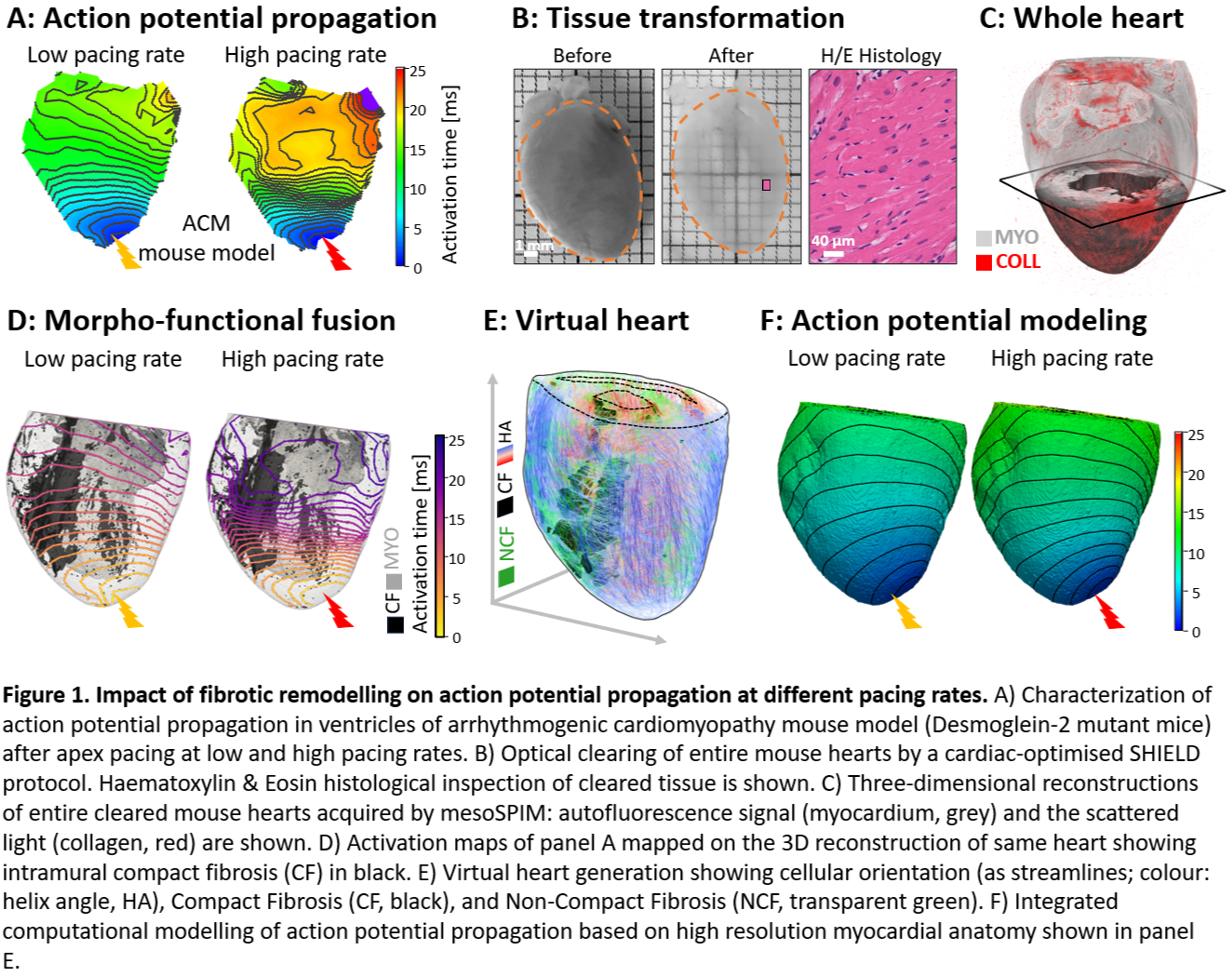
Introduction: Cardiac fibrosis is recognised as one of the key factors contributing to electrical conduction disturbances. However, how various forms of fibrosis affect conduction is uncertain, hindering predictive concepts regarding cardiac electrophysiology and arrhythmogenesis. In particular, the interplay between structural remodelling and electrical behaviour in fibrotic tissue remains poorly understood.
Methods: We implemented a combination of advanced imaging techniques and image analysis software tools to quantify and correlate macro-scale cardiac electrophysiology with 3D micro-scale structural reconstructions of whole ventricles in an arrhythmogenic cardiomyopathy (ACM) mouse model (Fig. 1A-D). Additionally, we generated a computational model of the electrical activity of representative hearts, combining myocardial anatomy, a fine quantification of 3D myocyte alignment, and detailed 3D fibrosis maps based on structural reconstructions (Fig. 1E-F).
Results: We characterised the dynamics of conduction wavefronts travelling through fibrotic regions, confirming that ACM involves a replacement of cardiomyocytes with fibrotic tissue, contributing to ventricular electrical dysfunction. Moreover, we observed that conduction through fibrotic areas shows a pacing-rate-dependent behaviour, where conduction failed at high stimulation frequencies, promoting reentrant arrhythmias. Importantly, we found that the dependency on the pacing rate of conduction through fibrotic areas cannot be explained solely by structural remodelling of the tissue (neither including fibrosis nor altered cardiomyocyte organisation).
Conclusion: These findings suggest that an accurate prediction of conduction disturbances requires consideration of electrophysiological remodelling of the myocardium and/or heterocellular interactions. Taken together, this study describes a new pro-arrhythmogenic aspect of cardiac electrophysiology in fibrotic tissue, namely the frequency-dependence of conduction, highlighting the dynamic functional nature of conduction block in the presence of myocardial fibrosis, the need for dynamic protocols to characterise conduction, and the desirability of further exploration of underlying (hetero-)cellular electrophysiological mechanisms.
Funding: This research project has been supported by the by the German Research Foundation (DFG) under grant #502822458 to LS and CZJ, and by DGK Research Grant of the German Society for Cardiology – Cardiovascular Research to FG.