BackgroundCompared to human cMRI, quantitative assessment in small animals is challenging for several reasons. Firstly, the ultra-high field strengths of preclinical scanners pose high demands on calibration steps and artifact correction. Furthermore, the acceleration of MR sequences is hampered as parallel imaging is limited due to the reduced multi-channel-coil options and advanced readouts such as spiral are hardly feasible
1. Therefore, it is necessary to optimize reconstruction pipelines of undersampled MRI data for maximum efficiency.
In this study, we transferred a novel low-rank-reconstruction technique developed for human MR fingerprinting
2 to highly undersampled MR data obtained from cMRI experiments in mice. The aim of this work was to realize myocardial T
1ρ quantification with 8-fold acceleration.
MethodsThe measurements were performed on a 7T preclinical MRI system (Bruker PharmaScan) with a single-channel quadrature transmit-receive birdcage coil. Data of an 8-fold accelerated T
1ρ mapping experiment with radial sampling pattern were analyzed, including one healthy animal and one with myocardial infarction. The results of undersampled reconstruction (UR) were compared with results of the low-rank reconstruction (LRR). The algorithm of LRR iterates a Dai-Yuan conjugate gradient descent
2 and finally leads to a model-based solution, which implies the exponential T
1ρ decay as a physical constraint. The thus quantified T
1ρ and R
2 values were compared with UR in the left ventricle.
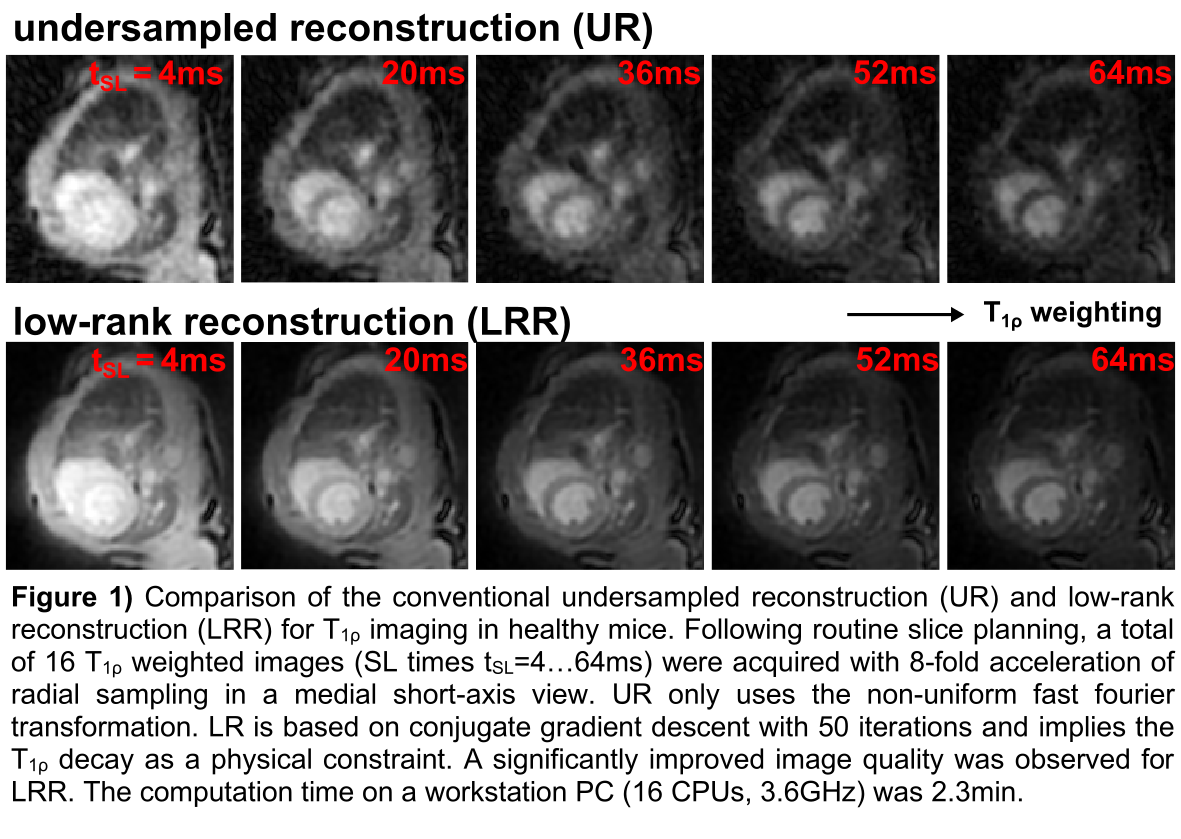
ResultsFig. 1 depicts the direct comparison of UR and LRR for exemplary T
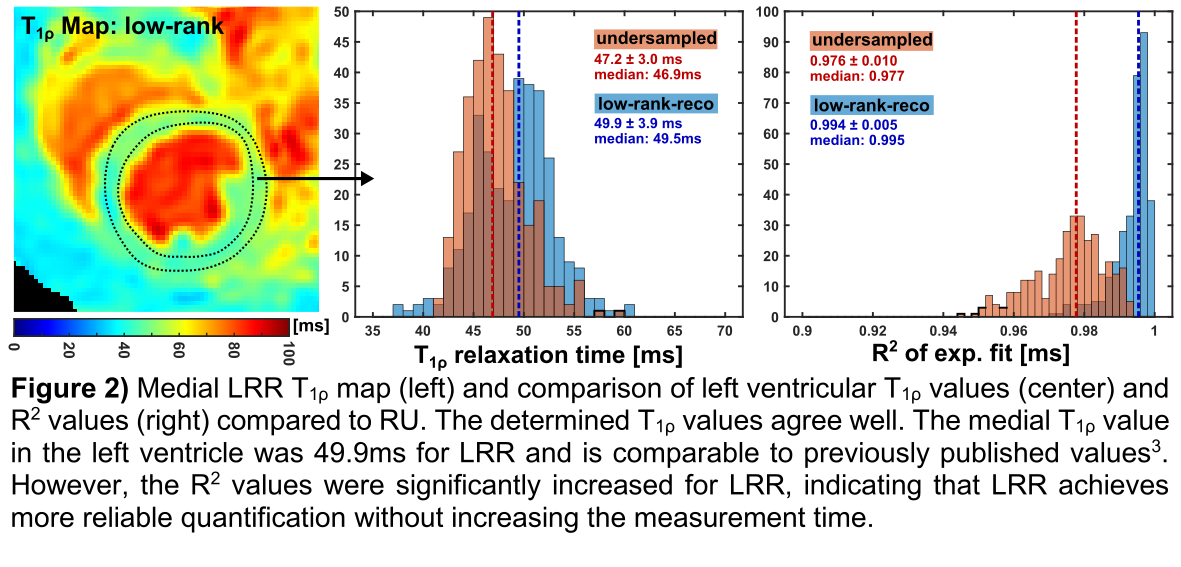
1ρ weighted images. A significant improvement in image quality (signal-to-noise-ratio: LRR 21.2, UR 8.4) is evident and LRR is nearly free of undersampling artefacts. Fig. 2 shows the T
1ρ map of the healthy animal and its evaluation in the left ventricle. The mean T
1ρ deviation compared to the values obtained with UR is 1.8±3.3ms. However, significantly increased R
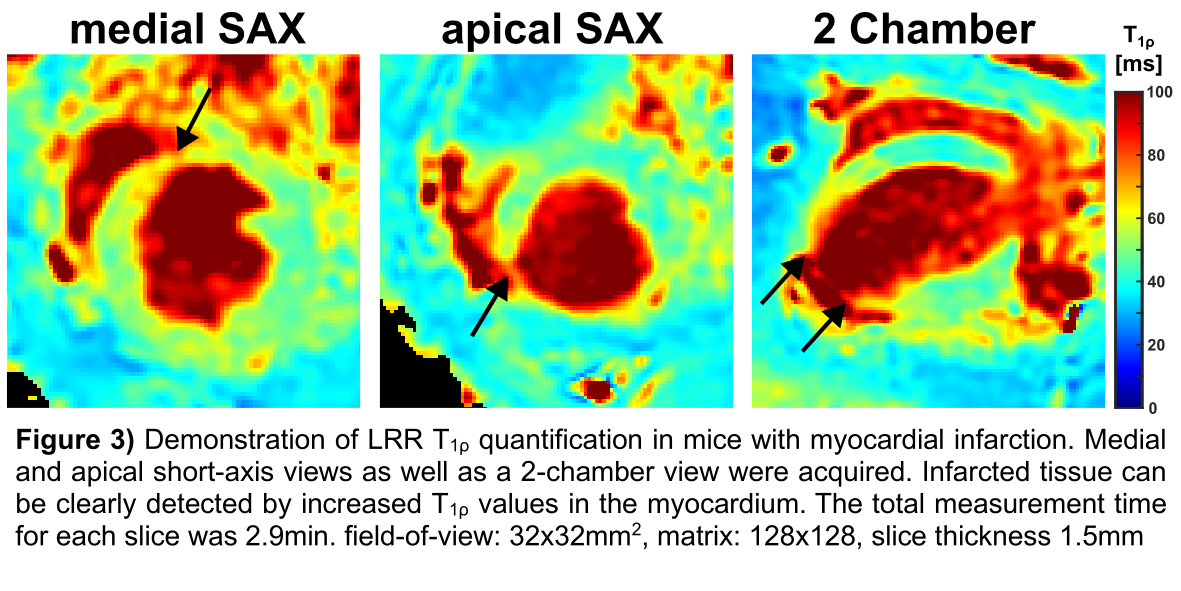
2 values were obtained for LR (0.994±0.005 vs. 0.976±0.010). The results of the MI study (Fig. 3) demonstrate that LRR can clearly differentiate infarcted tissue from remote tissue, whereby increased T
1ρ values were observed.
DiscussionIn this work, 8-fold accelerated quantification of the T
1ρ relaxation time was successfully achieved using LRR. Compared to UR, a diagnostic image quality was generated without the need for k-space view-sharing approaches
3, which are known for both miss-quantification and edge blurring/sharpening effects. The key to this efficient method is that low-rank implies a physical model for the reconstruction process. Our presented work is the first step to implement highly efficient multiparametric acquisitions for preclinical cMRI. In future studies, we plan to use LRR for fingerprinting sequences to achieve simultaneous and co-registered T
1, T
2 and T
1ρ maps in short acquisition times. Another important step will be the upgrade from radial to spiral acquisitions to achieve further efficiency gains
4.
[1] Gu Y, et al. doi: 10.1002/mrm.27345
[2] Hamilton JI, et al. doi: 10.1002/nbm.4041
[3] Gram M, et al. doi: 10.1007/s10334-021-00951-y
[4] Scholten H, et al. doi: 10.1002/nbm.5249
AcknowledgementsThe authors would like to thank Tom Griesler and Jesse Hamilton (Seiberlich Lab, University of Michigan) for the initial support with the LRR, which was adapted from human cMRI studies
2. We would also like to thank the Ramos Lab (University Hospital Würzburg) for the preparation of the MI model.