Introduction: Optogenetic defibrillation offers a novel strategy for terminating cardiac arrhythmias by utilising light-gated ion channels, known as channelrhodopsins (ChR). Research has primarily focused on cation non-selective ChR, such as Chlamydomonas ChR-2 (ChR2), and anion non-selective variants, like Guillardia theta Anion ChR-1 (GtACR1).[1] However, these channels depolarise resting cardiomyocytes (CM), potentially leading to intracellular Ca2+ accumulation – an effect that may exacerbate arrhythmias. In contrast, K+-selective ChR, such as Wobblia inhibitory ChR (WiChR),[2] could provide a safer alternative by maintaining CM near their resting potential.
Methods: We developed a first computational model of WiChR, based on single-cell patch-clamp recordings and fluorescent measurements of [K+]i in ND7/23 neuronal cells. To theoretically simulate human ventricular CM behaviour, we used the ten Tusscher model,[3] and integrated either our WiChR model, or established models of ChR2[4] or GtACR1.[1] Diastolic potential and [Ca2+]i were analysed as the minimal value in the last 0.5 s of illumination. Organ scale electrophysiological simulations were performed using openCARP.[5] Here, three geometries of human left ventricles, which were reconstructed from patients with ischaemic cardiomyopathy, were used.[6] Re-entry was induced by rapid pacing. Illumination from both the epi- and endocardium was simulated as a constant irradiance on the surface and exponential decay of light intensity in the tissue.[1] We tested three different start times of 1 s light application per patient geometry, yielding nine defibrillation attempts in total.
Results: In single cell simulations with 1 s illumination, diastolic CM potential approached –74 mV, –40 mV, and –41 mV, during activation of WiChR, GtACR1, and ChR2, respectively (Fig. 1A). Diastolic [Ca2+]i during illumination depended on the ChR variant (0.11 µM, 0.16 µM, and 0.16 µM for WiChR, GtACR1, and ChR2, respectively). In electrophysiological simulations of human left ventricles, WiChR-activation for 1 s with 0.5 mW/mm2 surface irradiance terminated re-entry in 7/9 cases, whereas the success rates of GtACR1 and ChR2 were 7/9 and 1/9, respectively. We identified three underlying mechanisms: (1) WiChR and GtACR1 fully inhibit action potentials when the local irradiance is larger than 0.005 mW/mm2, (2) rotors may drift under subthreshold illumination, and (3) GtACR1 may trigger new wavefronts that can give rise to new re-entry (Fig 1B).
Conclusion: Our results suggest that ChR that avoid pronounced depolarisation of CM, such as WiChR, may be safer for optogenetic defibrillation. In the future, we will test intermittent light pulses and patterned illumination, and validate our theoretical predictions experimentally.
Ochs AR et al. Front Physiol 2021/12:718622
Vierock J et al. Sci Adv 2022/8:eadd7729
Ten Tusscher KHWJ et al. Phys Med Biol 2006/23:6141
Wülfers EM et al. CinC 2018/45:1
Planck G et al. Comput Meth Prog Bio 2021/208:106223
Mendonca Costa C et al. Heart Rhythm 2019/16:1475
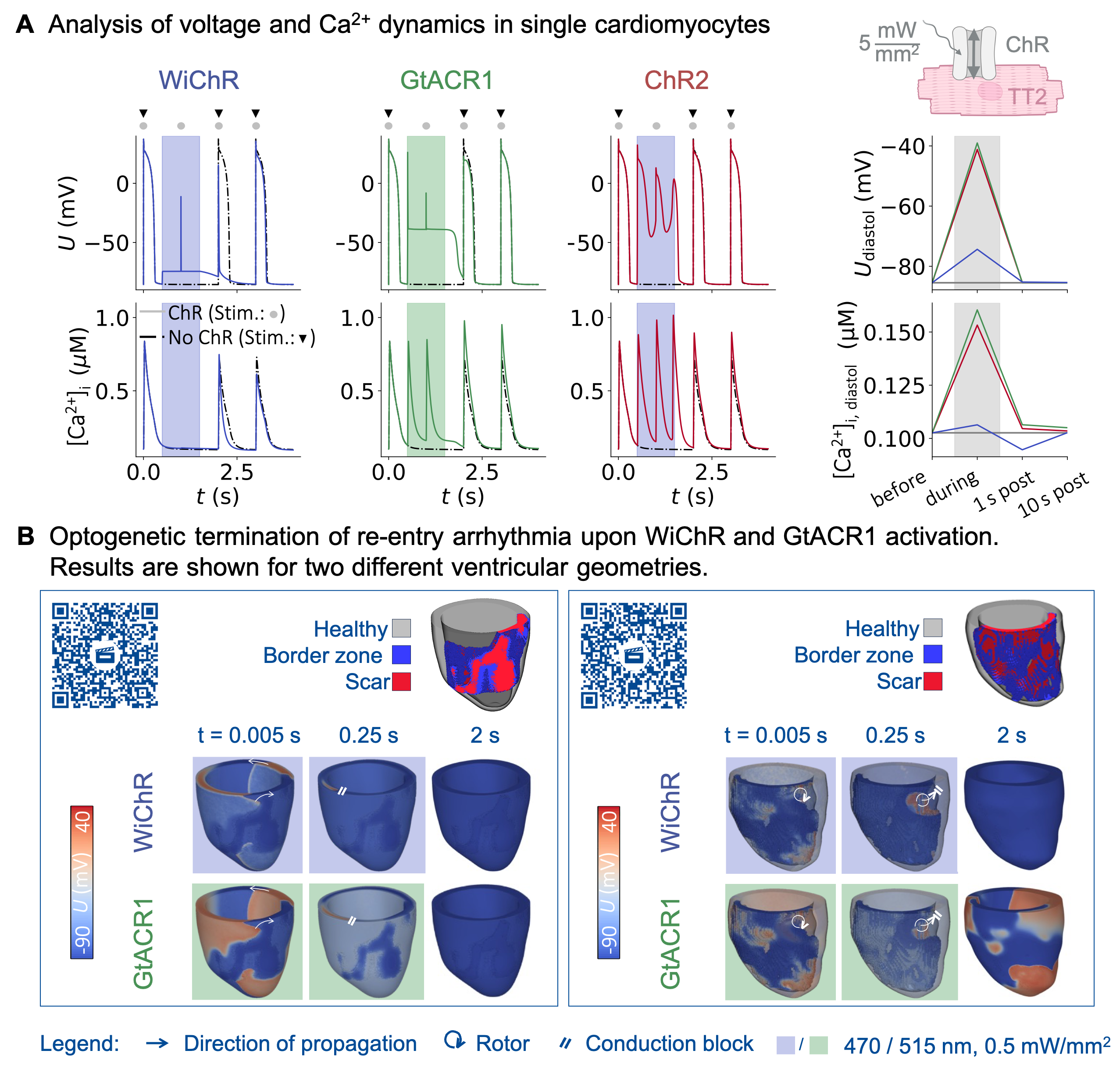
Fig. 1: (A) Simulated effects of WiChR, GtACR1, and ChR2 on CM potential U (top), and [Ca2+]i (bottom). Diastolic values (right) are shown before, during, as well as 1 s and 10 s post illumination. (B) In-silico assessment of WiChR and GtACR1-mediated defibrillation of re-entry arrhythmia in human left ventricles. Bar codes can be used to view time-resolved videos.
