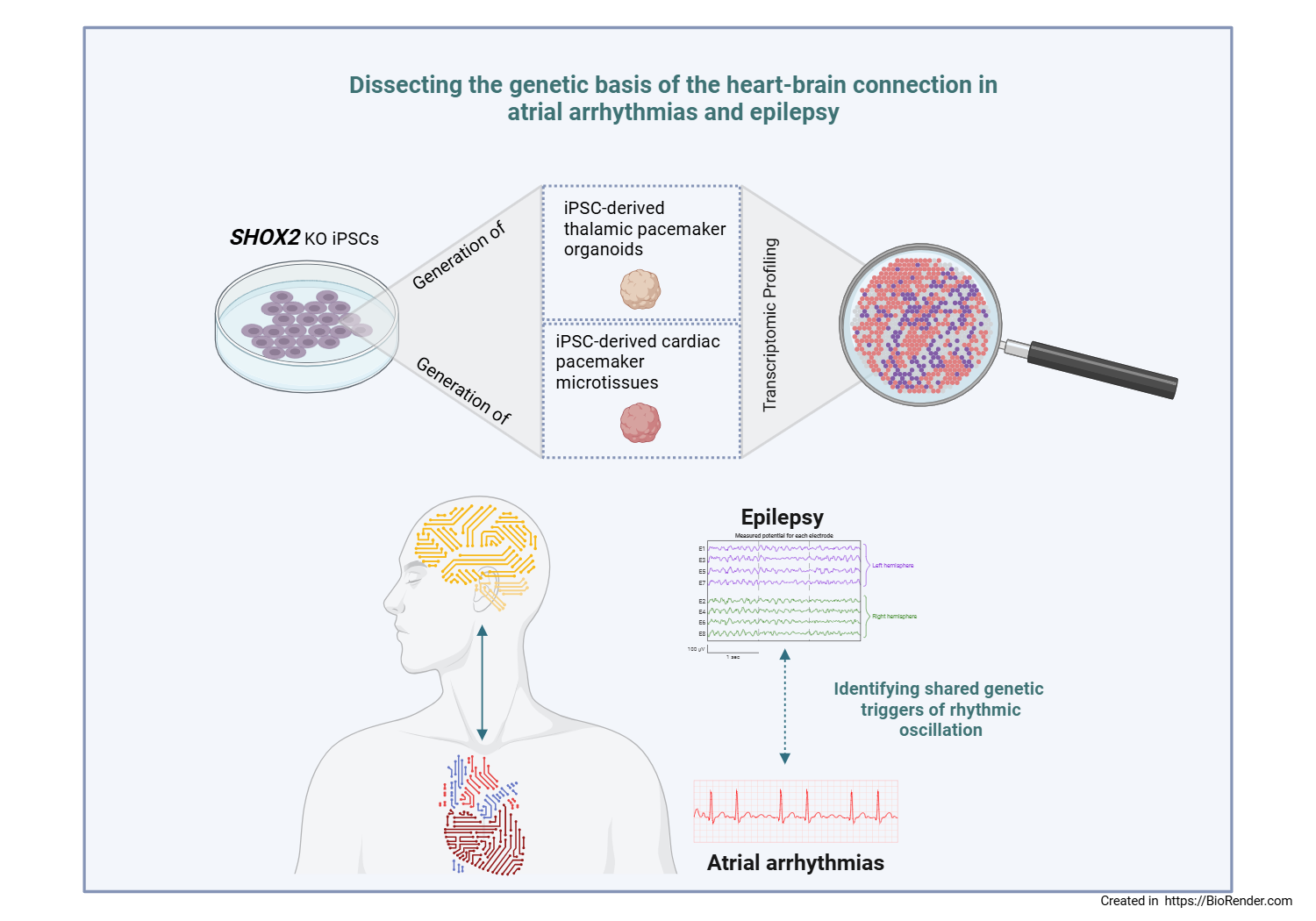
The sinoatrial node (SAN) is a cluster of specialized cardiomyocytes generating spontaneous action potentials to initiate each heartbeat. Rhythmic oscillations in the brain arise from interactions among the thalamus, the inhibitory thalamic reticular nucleus, and the cortex. The transcription factor Shox2 is essential for SAN development and the regulation of ion channels in thalamocortical neurons in mice, with its deficiency leading to cardiac arrhythmias and increased susceptibility to epilepsy. In humans, SHOX2 variants are associated with atrial fibrillation and sinus node dysfunction. Shared genetic mechanisms may underlie rhythmic oscillations in both heart and brain.
This study aims to generate and characterize SHOX2 knockout human induced pluripotent stem cell (hiPSC)-derived thalamic organoids and SAN microtissues, with the goal of uncovering shared regulatory networks that control pacemaker activity in the heart and brain.
We utilized homozygous SHOX2 knockout hiPSCs and patient-derived hiPSCs carrying heterozygous SHOX2 variants, along with their isogenic controls. Small-molecule directed differentiation was applied to generate SAN cardiomyocytes. 3D cardiac pacemaker microtissues were formed by aggregating 70% SAN cardiomyocytes, 15% cardiac microvascular endothelial cells, and 15% cardiac fibroblasts into 10,000-cell spheroids. Thalamic organoids were generated from hiPSCs using a static-to-spinning small-molecule protocol. Organoids and microtissues were characterized by immunofluorescence staining and qPCR, revealing characteristic molecular features. Both models expressed HCN family members, indicating shared pacemaker components. Pacemaker 3D microtissues displayed enhanced structural and functional maturation relative to 2D monolayer SAN cardiomyocytes, evidenced by increased GJA1 and adult SCN5A isoform expression. Cardiac contractility was assessed under various treatment conditions (e.g. with phenylephrine or Mavacamten), demonstrating distinct functional responses. Finally, comparative transcriptomic profiling of SHOX2 knockout and isogenic control organoids and microtissues identified shared regulatory networks, revealing deregulation of several ion channels in both the heart and brain. These findings suggest a potential role for SHOX2 in coordinating rhythmic activity across cardiac and neuronal pacemaker systems.
Our study demonstrates that SAN microtissues exhibit enhanced maturation and, together with thalamic organoids, uncover common SHOX2-dependent regulatory networks in the heart and brain. SHOX2 orchestrates pacemaker-specific transcriptional programs and modulates key ion channels in both cardiac and neuronal iPSC-derived tissues. These results position SHOX2 as a central coordinator of heart–brain pacemaker activity and establish a versatile human iPSC-based platform for investigating arrhythmia mechanisms and seizure-related cardiac dysfunction.
Unraveling shared SHOX2-dependent molecular pathways may provide potential biomarkers for the early identification of epilepsy patients at risk for cardiac arrhythmias and sudden unexpected death in epilepsy (SUDEP). Clinical data indicate that up to 25 % of epilepsy patients experience cardiac arrhythmias, contributing to an elevated SUDEP risk. Regulatory networks controlling pacemaker activity in the heart and brain could reveal common pathomechanisms, genetic disease triggers, and novel drug targets to prevent cardiac arrhythmias in epilepsy patients.
