Background:
Hospitalization is a significant clinical event in the progression of cardiac transthyretin (ATTR) amyloidosis, but not all patients (pts) who worsen become hospitalized. Outpatient oral diuretic intensification or diuretic initiation (ODI) for heart failure (HF) has been shown to be prognostic of outcomes in pts with HFrEF or HFpEF.
Methods:
This post hoc analysis of APOLLO-B (NCT03997383; comparing patisiran vs pbo in ATTR cardiomyopathy pts in a 12-month double-blind [DB] period followed by an open-label extension [OLE], in which all pts receive patisiran) assessed the effect of patisiran on ODI and a composite endpoint of all-cause mortality, cardiovascular (CV) events, and ODI by a win-ratio method.
Results:
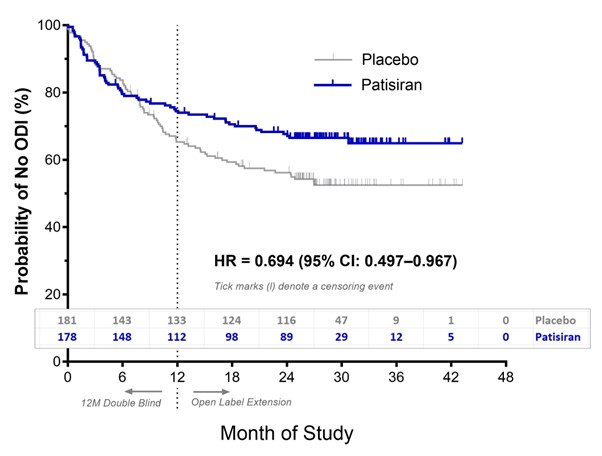
In APOLLO-B (combined DB and OLE with all remaining pts having reached Month 24 or later), for patisiran vs pbo (per initial treatment in DB), 61 (33.7%) vs 81 (45.5%) had outpatient ODI, 88 (48.6%) vs 93 (52.2%) had a CV event, and 19 (10.5%) vs 28 (15.7%) died. In Kaplan-Meier analysis, the probability of freedom from ODI was higher with patisiran vs pbo with separation of the two arms during the DB period (HR 0.694; 95% CI: 0.497–0.967) (Figure). Patisiran was associated with a win ratio of 1.31 (95% CI: 0.97–1.75) on the composite endpoint, reflecting more favorable outcomes than pbo.
Conclusions:
In APOLLO-B, worsening HF requiring ODI was significantly reduced by patisiran. Patisiran had a favorable effect on the combined risk of all-cause mortality, frequency of CV events, and ODI.