Background
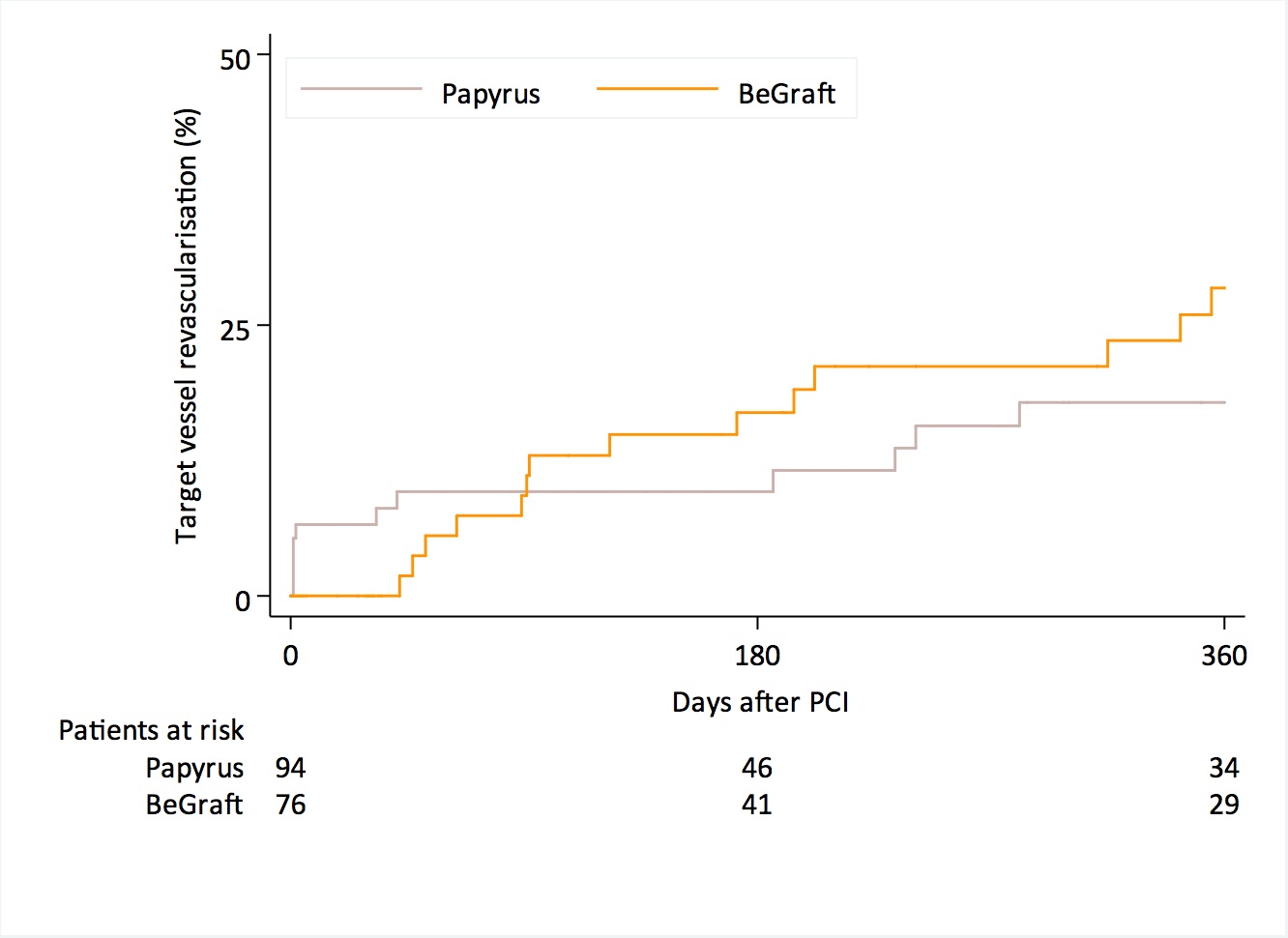
New-generation single-layer polytetrafluorethylene (PTFE-) or polyurethan (PU-) covered stent (CS) for the treatment of coronary artery perforation (CAP) during PCI offer high procedural efficacy.
Aim
To evaluate the comparative long-term safety and efficacy of both devices.
Methods
This is a multicenter pooled analysis of individual data of patients with CAP undergoing implantation of single-layer PTFE-CS or PU-CS (Figure1). Procedural endpoint was, strategy success defined as successful placement of CS and sealing of perforation without surgical conversion.
Clinical endpoints were mortality, myocardial infarction (MI), target vessel revascularization (TVR) and definite or probable stent thrombosis (def/prob ST) at 12 months.
Results
170 patients with CAP underwent implantation of 208 CS, 92 PTFE-CS and 116 PU-CS. More than one stent was implanted in 13 patients (17.1%) in PTFE-CS group and 19 patients (20.2%) in PU-CS group, p=0.80. Strategy success was high (96.1% versus 92.5%., P=0.62).
At 12 months 71 patients (93.2%) in PTFE-CS group versus 79 patients (81%) in the PU-CS were alive, P=0.05, TVR occurred in 14 patients (28.4%) in PTFE-CS group and 12 patients (17.9%) in PU-CS group, p=0.54 (Figure 2 ); MI in 1 patient (1.3%) in PTFE-CS group and 1 patients (1.1%) in PU-CS group, p=0.86. Rates of def/prob ST were comparable 1.3% in PTFE-CS versus 3.1% in PU-CS P=0.95.
Conclusions
A strategy of implantation of a new generation single layer PTFE- or PU-CS for the treatment of coronary artery perforation showed high success rates. Both new generation CS showed favourable and similar clinical safety, in particular with regard to thrombotic events.
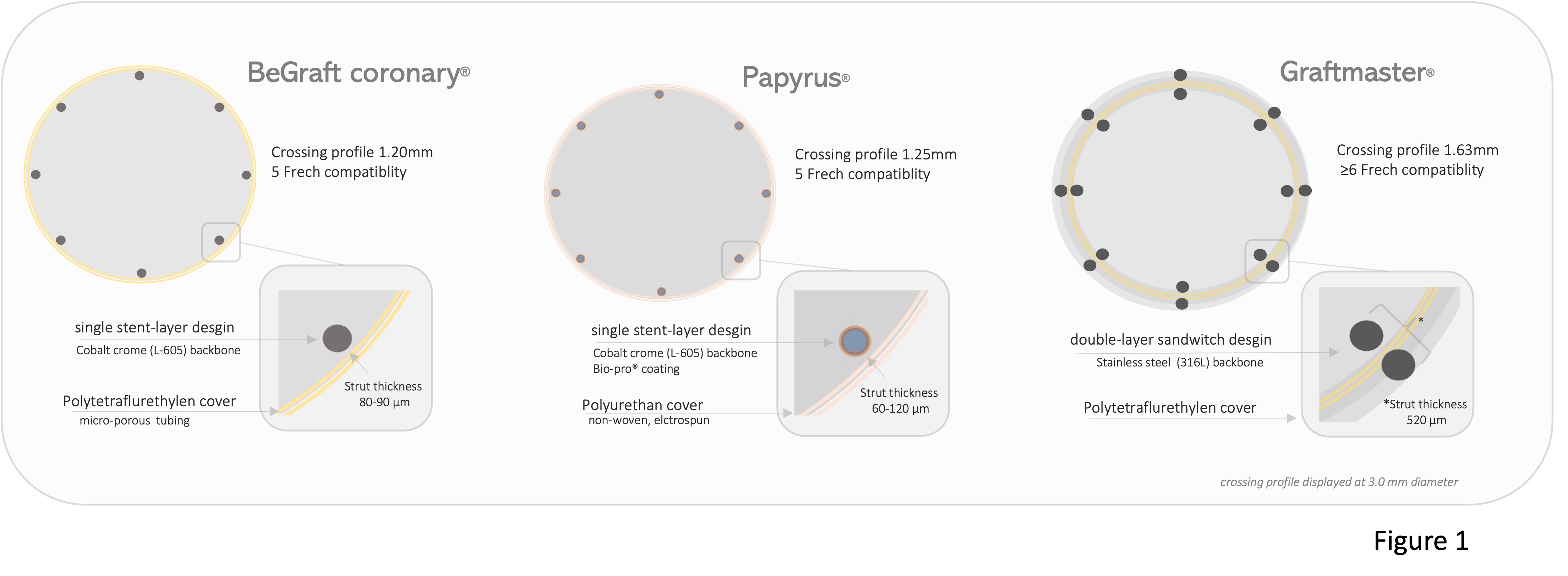
Figure 2