Background
Atrial arrhythmias (AAs) in patients with left ventricular cardiomyopathy (LV-CM) are frequent and manifest at a younger age than in the general population. Different (likely) pathogenic variants (LP/PVs) in cardiomyopathy related genes are associated with variable penetrance, disease expression and progression which may be relevant for AA substrates. Data on AA ablation outcomes in patients with an LV-CM phenotype are sparse and data on genotype-specific ablation outcomes in this cohort are currently lacking. The aim of this study was to analyze outcomes of CA in LV-CM patients in relation to different genotypes.
Methods
Patients who underwent CA for non-CTI dependent AAs and genetic testing for pathogenic variants in CM-associated genes (n=72, 96% with structural LV abnormalities; n=3, 4% tested during family screening) were included. Genetic testing results, ablation outcomes (one year AA-free survival) and clinical outcomes (echocardiography parameters at one year follow up) were analyzed across subgroups based on genetic findings (LP/PV-negative, TTN, LMNA, and other LP/PV).
Results
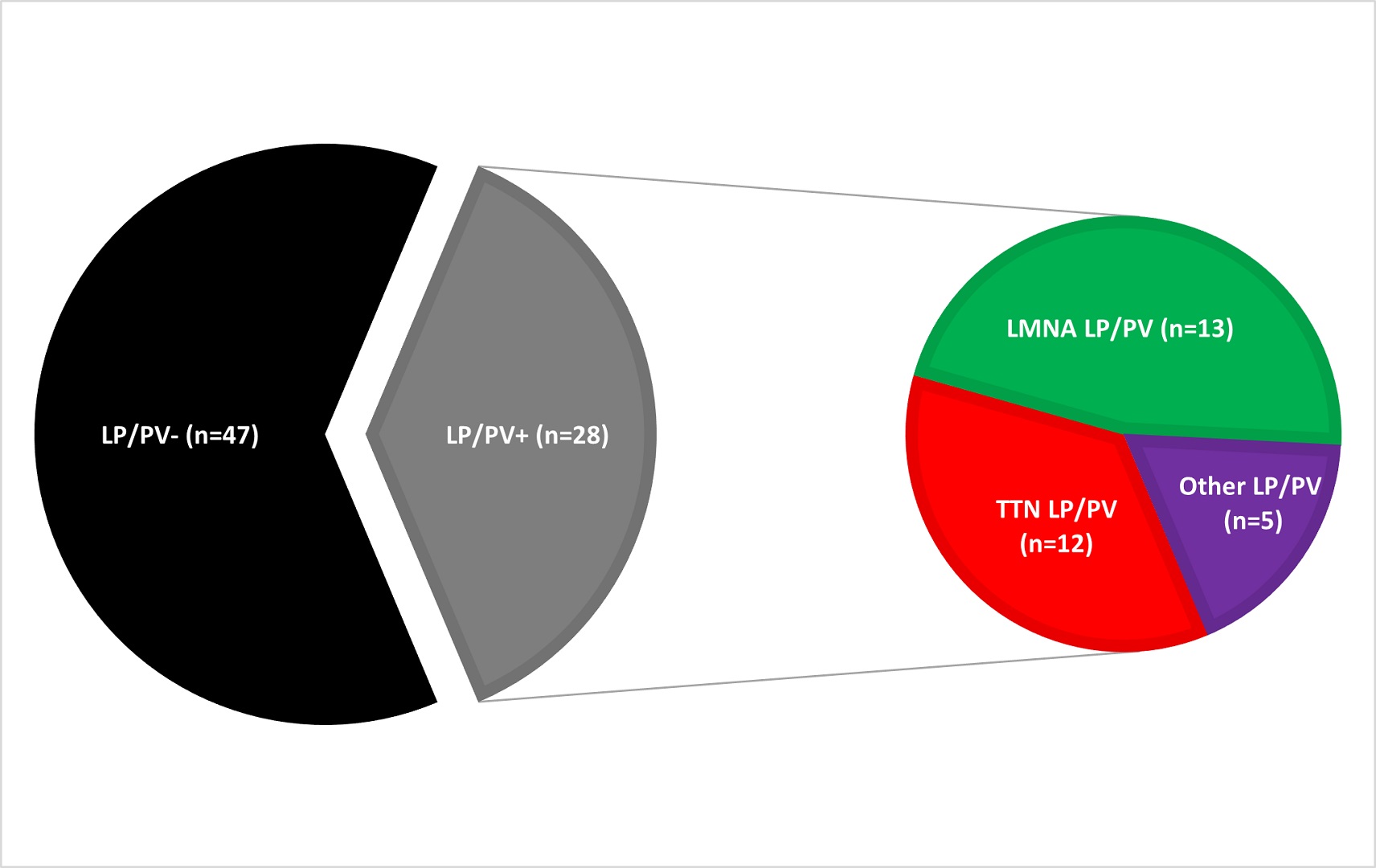
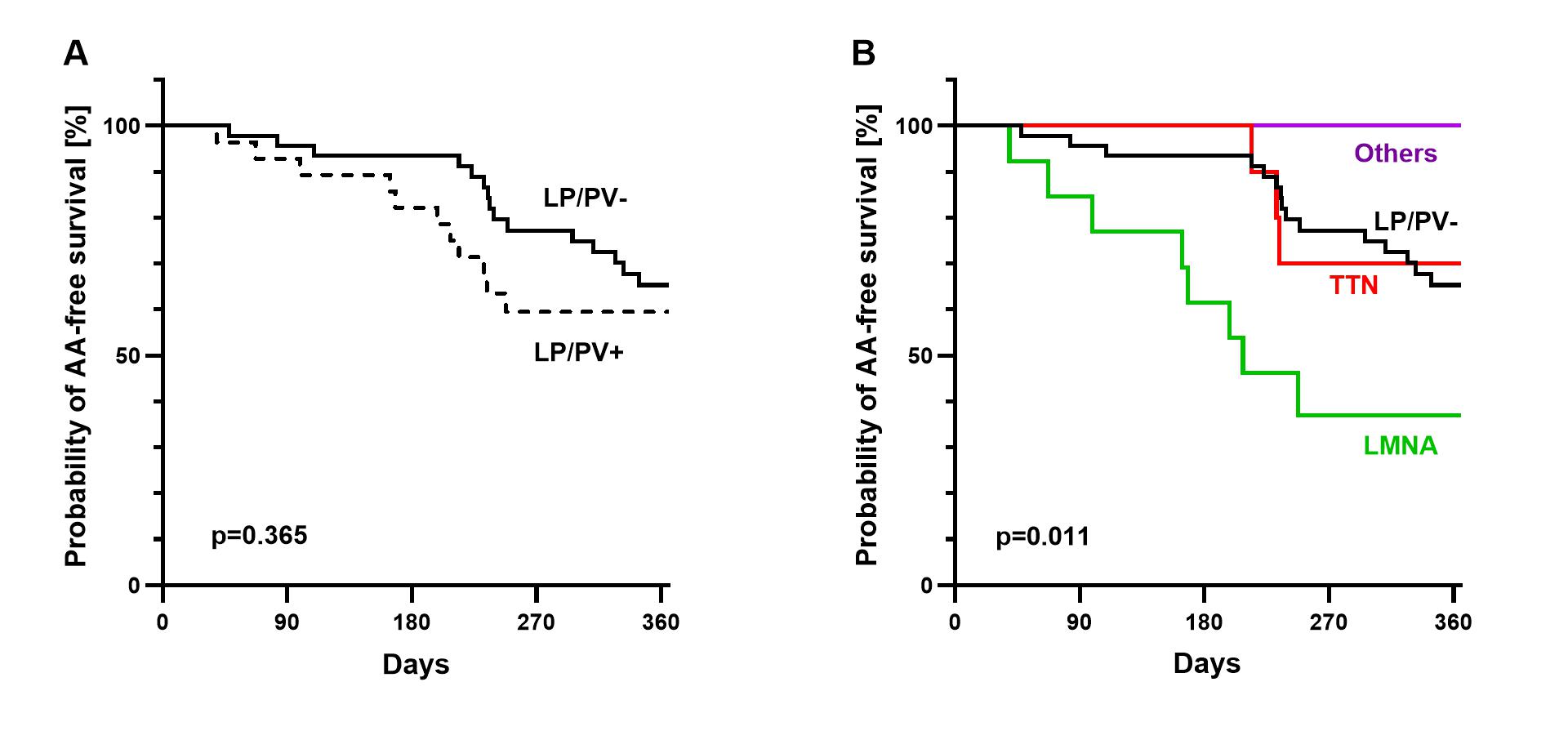
Seventy-five consecutive patients (age 58 ± 13 years, 76% male, mean LV-EF 45.8% ± 11%) who underwent CA for AAs were included (paroxysmal AF: n=24, 32%; persistent AF: n=40, 53%; AF only: n=46, 61%; AF + atypical flutter: n=8, 11%; atypical flutter only: n=11, 15%). A LP/PV was found in 28 (37%) patients (LMNA: n=13/28, 46%; TTN: n=10/28, 36%; Other LP/PV: n=5/28, 18%) (Figure 1). One-year AA-free survival did not differ between patients with and without LP/PV [LP/PV-: 65%, 95% CI (51% to 80%) vs. LP/PV+: 60%, 95% CI (41% to 78%), Log Rank=0.365] (Figure 2A). In a subgroup analysis, LMNA patients had a higher chance of 1-year AA recurrence compared to LP/PV- [HR=3.081, 95% CI (1.296-7.325)] while patients with a TTN LP/PV did not [HR=0.899, 95% CI (0.26-3.106)] (Figure 2B). In a univariable analysis, an LMNA LP/PV, AV block at baseline, LA/RA low-voltage areas were predictors of AA recurrence (p<0.1). In a multivariable analysis, an LMNA LP/PV was the only independent predictor [HR: 3.835, 95% CI (1.296-11.351), p=0.015]. At 1 year FU, left ventricular ejection fraction (LV-EF) significantly improved in TTN patients (BL 44% ± 9.2 vs. FU 48.8% ± 8.5, p=0.022), while no significant changes were observed for the other subgroups (LP/PV-: BL 45.4% ± 10.5 vs. FU 48.5% ± 8.7, p=0.063; LMNA: BL 52% ± 9.2 vs. FU 50% ± 11.8, p=0.296; Other LP/PV: BL 37.1% ± 8.4 vs. FU 39.3% ± 7.9, p=0.161).
Conclusion
In LV-CM patients undergoing CA for AAs, LMNA LP/PVs were associated with higher recurrence rates post-ablation. In contrast, patients with TTN LP/PVs did not have a higher recurrence rate than patients without LP/PVs and, importantly, they showed improvement in LV function after ablation. These findings may inform patient selection and support the development of genotype-tailored treatment strategies.