1Uniklinik RWTH Aachen Med. Klinik I - Kardiologie, Angiologie und Internistische Intensivmedizin Aachen, Deutschland; 2UZ Leuven Department of Nephrology Leuven, Belgien; 3Uniklinik RWTH Aachen Institut für Molekulare Herz-Kreislaufforschung (IMCAR) Aachen, Deutschland; 4Uniklinik RWTH Aachen Interdisciplinary Center for Clinical Research (IZKF) Genomics Facility Aachen, Deutschland
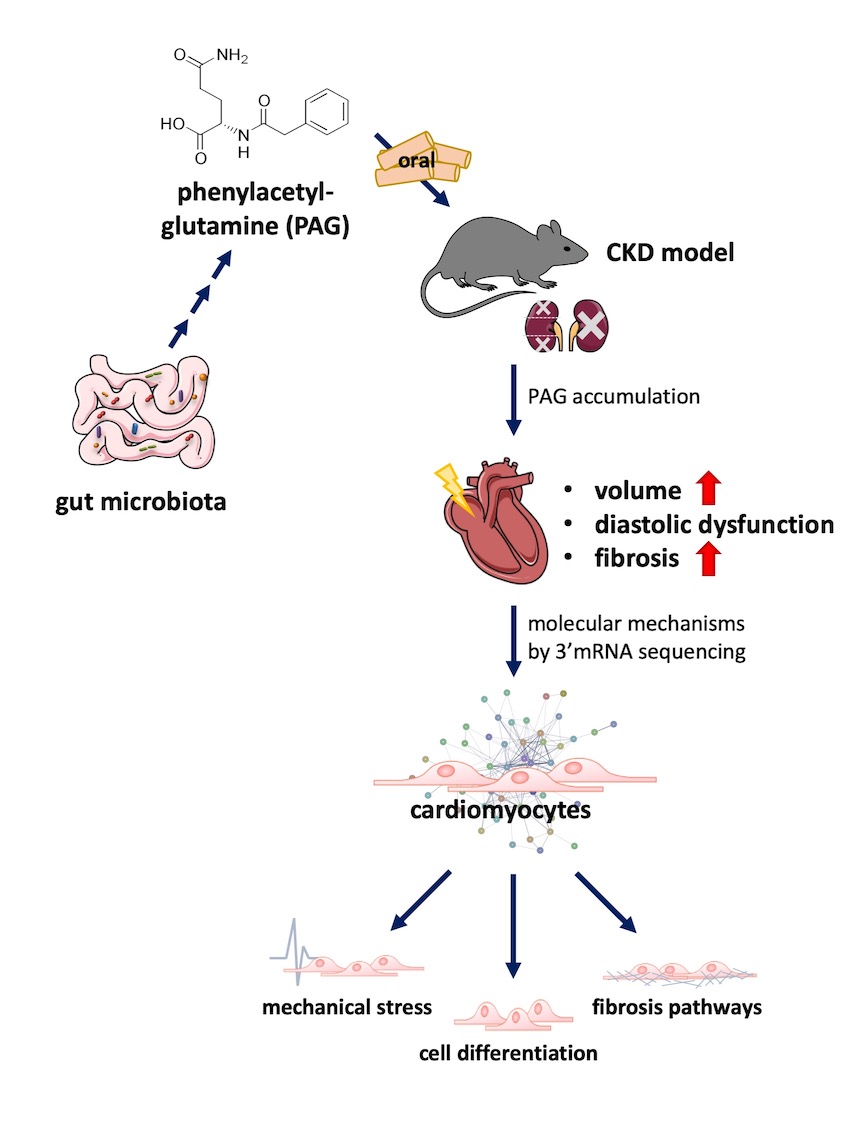
Background: Cardiovascular disease is the main cause of death in patients with moderate to severe chronic kidney disease (CKD). Within the paradigm of gut-heart-kidney cross talk, the pivotal role of gut microbiota and their metabolites has emerged as a focal point in understanding the intricate communication between organs. The gut-derived serum metabolite phenylacetylglutamine (PAG) has formerly been associated with mortality and cardiovascular disease in patients with CKD. In this study, we examined the influence of PAG on cardiac remodeling in a mouse model of CKD and unraveled molecular pathways that underlie the effect of PAG on the heart.
Methods: The impact of PAG on cardiac physiology was investigated in mouse models and HL-1 cardiomyocytes. Pharmacokinetic studies of PAG were conducted in C57Bl/6J mice with normal renal function and in mice subjected to 5/6-nephrectomy (5/6-Nx). Subsequently, PAG was orally administered in C57Bl/6J mice, with and without 5/6-Nx for a 12-week duration. Cardiac function and morphological changes were assessed through echocardiography and histological examinations. Gene expression profiles were studied using RT-PCR. To uncover underlying molecular mechanisms by which PAG affects cardiac remodeling, 3' mRNA sequencing was performed in HL-1 cardiomyocytes.
Results: Long-term administration of PAG did not affect cardiac function in mice with normal renal function. In our pharmacokinetic studies, rapid renal clearance of PAG was observed, whereas reduced kidney function in mice subjected to 5/6-Nx led to an accumulation of PAG in serum (7.4x increase of PAG in serum after 2 weeks (p<0.001) and 4x increase after 4 weeks (p<0.05) of PAG supplementation). Long-term supplementation of PAG in 5/6-Nx mice for 12 weeks significantly enlarged left ventricular volume measured by echocardiography 7 weeks after initiation of treatment (systolic volume: +35.0 ± 16.0%, p<0.05 and diastolic volume: +25.7 ± 9.9%, p<0.05), while changes in volume were not apparent anymore after 11 weeks. While left ventricular ejection fraction remained unaltered in 5/6Nx mice treated with PAG, heart-to-body weight ratio (+20.2 ± 7.9%, p<0.05) as well as diastolic dysfunction (i.e. shown by E/A ratio: +62.3 ± 13.7%, p<0.001) significantly increased. Based on histological and gene expression analysis, enhanced left ventricular fibrosis was observed in mice subjected to PAG (left ventricle fibrosis: +27.3 ± 8.7%, p<0.05 and Col1a1 mRNA expression: +29.5 ± 7.5%, p<0.01). To understand underlying molecular mechanisms of PAG in the heart, we performed 3’mRNA sequencing in HL-1 cardiomyocytes. PAG significantly (adjusted p<0.05) upregulated 100 and downregulated 8 genes. Pathway analyses revealed that these genes were linked to TGF-beta signaling, cardiac fibrosis as well as mechanical stress response.
Conclusion: The gut-derived metabolite PAG accumulates in CKD and negatively affects cardiac remodeling characterized by increased fibrosis and diastolic dysfunction. Therefore, PAG could be a potential mediator in the gut-heart-kidney axis and may play a role in uremic cardiomyopathy.