https://doi.org/10.1007/s00392-025-02625-4
1Universitätsklinikum Gießen und Marburg GmbH Medizinische Klinik I - Kardiologie und Angiologie Gießen, Deutschland; 2Universitätsmedizin Göttingen Göttingen, Deutschland; 3Universitäres Herzzentrum Regensburg Regensburg, Deutschland; 4Herz- und Diabeteszentrum NRW Klinik für Thorax- und Kardiovaskularchirurgie Bad Oeynhausen, Deutschland; 5Herz- und Diabeteszentrum NRW E.& H. Klessmann-Institut f. kardiovask. Forschung Bad Oeynhausen, Deutschland; 6Ruhr-Universität Bochum Zelluläre und Translationale Physiologie Bochum, Deutschland; 7Universitätsklinikum Würzburg Institut für Pharmakologie und Toxikologie Würzburg, Deutschland
Background:
Heart failure is a life-threatening disease with a high incidence worldwide. Even today, treatment is still demanding and faces many challenges. Traditionally used positive inotropic drugs have various adverse effects, especially with long-term use, such as high arrhythmogenic potential and receptor sensitization. Omecamtiv-mecarbil (OM) aims to close this gap by improving cardiac function at the sarcomere level. So far, the mechanisms of OM on contractility and arrhythmogenicity in human ventricular tissue have been barely investigated. In particular, the subgroup of patients with low ejection fraction has been shown to benefit from OM in a clinical trial. This study aims to provide more clarity by specifically investigating the effects of OM on the myocardial tissue of donors with low ejection fraction.
Methods:
Human cardiac slices from left ventricular myocardium were freshly generated from patients with end-stage heart failure who underwent heart transplantation. Slices were long-term cultivated and separated into an OM treated and untreated control group, which were both stimulated over a 5 day period with 60 bpm.
Heart failure is a life-threatening disease with a high incidence worldwide. Even today, treatment is still demanding and faces many challenges. Traditionally used positive inotropic drugs have various adverse effects, especially with long-term use, such as high arrhythmogenic potential and receptor sensitization. Omecamtiv-mecarbil (OM) aims to close this gap by improving cardiac function at the sarcomere level. So far, the mechanisms of OM on contractility and arrhythmogenicity in human ventricular tissue have been barely investigated. In particular, the subgroup of patients with low ejection fraction has been shown to benefit from OM in a clinical trial. This study aims to provide more clarity by specifically investigating the effects of OM on the myocardial tissue of donors with low ejection fraction.
Methods:
Human cardiac slices from left ventricular myocardium were freshly generated from patients with end-stage heart failure who underwent heart transplantation. Slices were long-term cultivated and separated into an OM treated and untreated control group, which were both stimulated over a 5 day period with 60 bpm.
Additionally, experiments were conducted using human induced pluripotent stem cell cardiomyocytes (iPSC-CM). A heart failure phenotype was induced by stimulating cells with a tachycardic frequency of 120 bpm. Cells were divided into 3 groups: (1) control group (60 bpm), (2) tachycardic group (120 bpm) and (3) OM-treated group (120 bpm). After 48 hours and 7 days, epifluorescence microscopy was performed to measure calcium homeostasis using Fura-2-AM.
Slices and cardiomyocytes were treated with an OM concentration of 0.5 µM, based on preliminary experimental studies and plasma concentration observed in clinical trials.
Results:
After 5 days, a significant increase in twitch amplitude could be observed in human cardiac slices when the control group was compared to the OM group (figure 1). No relevant differences were identified in either diastolic tension, relaxation time or time to peak. Additionally, the amount of arrhythmia events was not higher in OM-treated slices in comparison to the control slices.
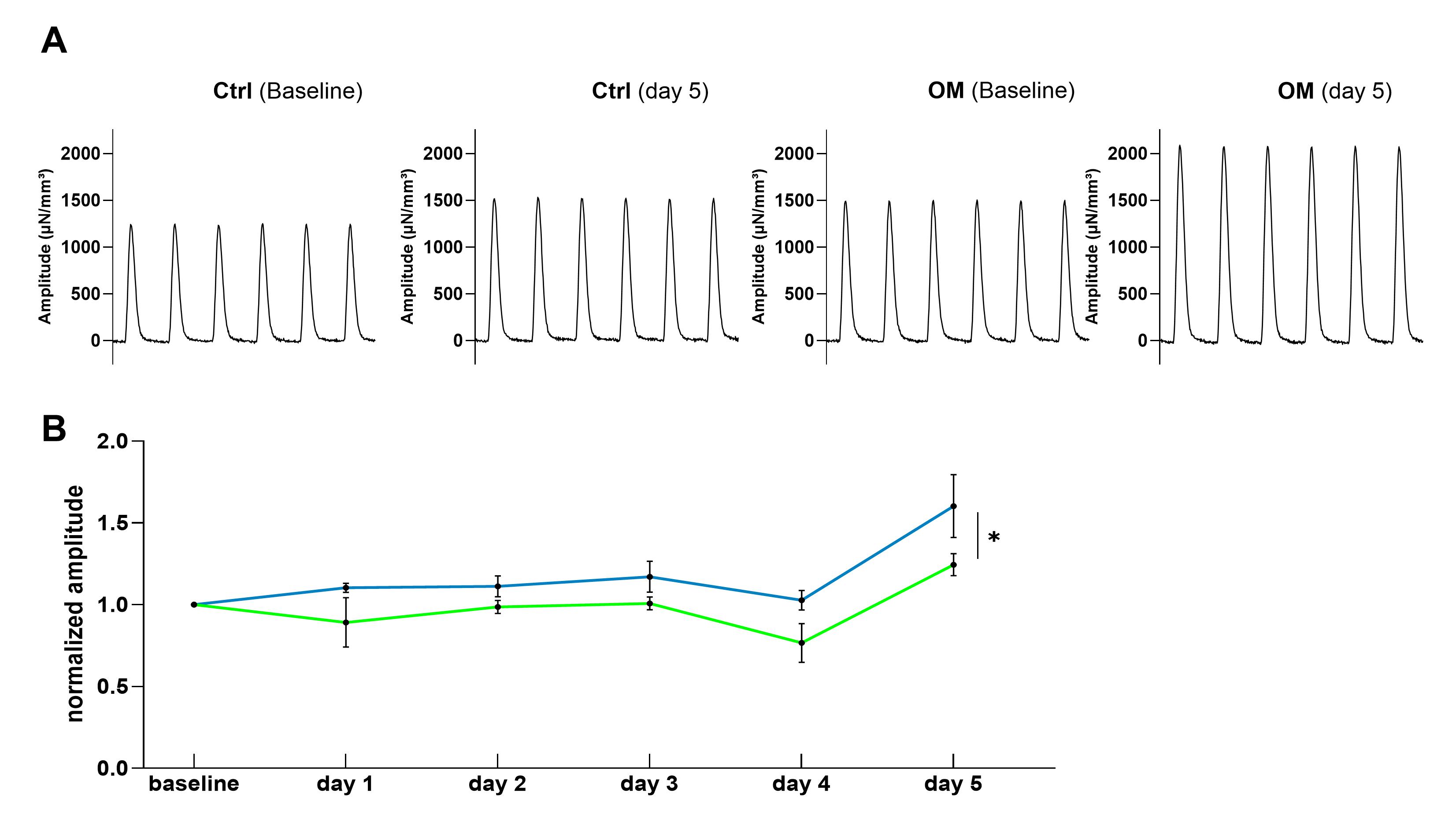
After 5 days, a significant increase in twitch amplitude could be observed in human cardiac slices when the control group was compared to the OM group (figure 1). No relevant differences were identified in either diastolic tension, relaxation time or time to peak. Additionally, the amount of arrhythmia events was not higher in OM-treated slices in comparison to the control slices.
Fig. 1: (A) Original traces of Ctrl and OM human cardiac slices at baseline and day 5 (B) Normalized amplitudes of human cardiac slices in comparison between OM group (blue) and control group (green); 10 slices from 2 hearts were used; a two-side ANOVA was used to test significance (* p<0.05); data are presented as mean ± SEM
In the iPSC-CMs, after 48 h epifluorescence microscopy revealed no significant differences in calcium transients, diastolic calcium levels, time-to-peak and relaxation time between the tachycardic group and the OM-treated tachycardic group. As expected, a tendency towards lower calcium transients in both tachycardic stimulated groups compared to the control group were detected.
Conclusion:
For the first time, we were able to show that OM leads to a marked improvement in contractility in human ventricular tissue from donors with low ejection fraction without increasing the occurrence of arrhythmias, thus confirming the clinical results. The next step is to investigate changes in myosin ATPase activity in human slices as a potential mechanism underlying the observed changes in contractile amplitude and to assess the effect of OM specifically on RV myocardium.
For the first time, we were able to show that OM leads to a marked improvement in contractility in human ventricular tissue from donors with low ejection fraction without increasing the occurrence of arrhythmias, thus confirming the clinical results. The next step is to investigate changes in myosin ATPase activity in human slices as a potential mechanism underlying the observed changes in contractile amplitude and to assess the effect of OM specifically on RV myocardium.