https://doi.org/10.1007/s00392-025-02625-4
Benedikt Köll (Hamburg)1, S. Ludwig (Hamburg)1, J. Weimann (Hamburg)1, L. Stolz (München)2, A. Scotti (New York)3, E. Xhepa (München)4, E. Donal (Rennes)5, D. Patel (Los Angeles)6, T. Tanaka (Bonn)7, T. Trenkwalder (München)4, F. Rudolph (Bad Oeynhausen)8, D. Samim (Bern)9, P. von Stein (Köln)10, C. Giannini (Pisa)11, J. Dreyfus (Paris)12, J.-M. Paradis (Quebec)13, M. Adamo (Brescia)14, N. Karam (Paris)15, Y. Bohbot (Amiens)16, A. Bernard (Tours)17, B. Melica (Espinho)18, O. De Backer (Copenhagen)19, Y. Lavie-Badie (Toulouse)20, M. Keßler (Ulm)21, C. Iliadis (Köln)10, S. Redwood (London)22, E. Lubos (Hamburg)23, M. Metra (Brescia)14, F. Praz (Bern)9, M. Gercek (Bad Oeynhausen)8, G. Nickenig (Bonn)7, T. Modine (Bordeaux)24, J. Hausleiter (München)2, A. Coisne (Lille)25, D. Kalbacher (Hamburg)26
1Universitäres Herz- und Gefäßzentrum Hamburg
Klinik für Kardiologie
Hamburg, Deutschland; 2LMU Klinikum der Universität München
Medizinische Klinik und Poliklinik I
München, Deutschland; 3Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine
New York, USA; 4Deutsches Herzzentrum München
Klinik für Herz- und Kreislauferkrankungen
München, Deutschland; 5Univ Rennes, CHU Rennes
Rennes, Frankreich; 6Smidt Heart Institute, Cedars-Sinai Medical Center
Los Angeles, USA; 7Universitätsklinikum Bonn
Medizinische Klinik und Poliklinik II
Bonn, Deutschland; 8Herz- und Diabeteszentrum NRW
Allgemeine und Interventionelle Kardiologie/Angiologie
Bad Oeynhausen, Deutschland; 9Inselspital - Universitätsspital Bern
Universitätsklinik für Kardiologie
Bern, Schweiz; 10Herzzentrum der Universität zu Köln
Klinik III für Innere Medizin
Köln, Deutschland; 11S.D. Emodinamica, AOUP - Azienda Ospedaliero Universitaria Pisana
Pisa, Italien; 12Cardiology Department, Centre Cardiologique du Nord
Paris, Frankreich; 13Quebec Heart & Lung Institute, Laval University
Quebec, Kanada; 14University of Brescia, Cardiac Catheterization Laboratory and Cardiology, ASST Spedali Civili and Department of medical and surgical specialties, radiological sciences and public health
Brescia, Italien; 15Cardiology Department, European Hospital Georges Pompidou
Paris, Frankreich; 16Department of Cardiology, Amiens University Hospital
Amiens, Frankreich; 17Cardiology Department, CHRU de Tours
Tours, Frankreich; 18Centro Hospitalar Vila Nova de Gaia
Espinho, Portugal; 19Rigshospitalet, Copenhagen University Hospital Copenhagen
Copenhagen, Dänemark; 20Department of Cardiology, Rangueil University Hospital
Toulouse, Frankreich; 21Universitätsklinikum Ulm
Klinik für Innere Medizin II
Ulm, Deutschland; 22Department of Cardiology, St. Thomas' Hospital
London, Großbritannien; 23Katholisches Marienkrankenhaus gGmbH
Kardiologie und Angiologie
Hamburg, Deutschland; 24Service Médico-Chirurgical: Valvulopathies-Chirurgie Cardiaque-Cardiologie Interventionelle Structurelle, Centre Hospitalier Universitaire Bordeaux
Bordeaux, Frankreich; 25Department of Clinical Physiology and Echocardiography - Heart Valve Clinic, CHU Lille
Lille, Frankreich; 26Universitäres Herz- und Gefäßzentrum Hamburg
Allgemeine und Interventionelle Kardiologie
Hamburg, Deutschland
Background
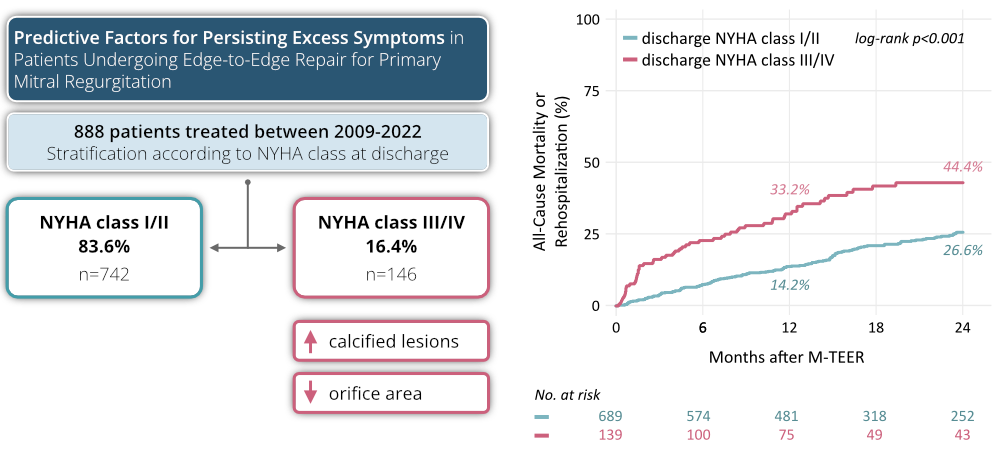
Residual mitral regurgitation (MR) post transcatheter edge-to-edge repair (M-TEER) is a strong indicator of adverse events in patients with primary MR (PMR). While M-TEER generally improves MR, a subgroup continues to experience a substantial symptomatic burden, shown by New York Heart Association (NYHA) class III or IV status at discharge. Identifying predictive factors for these persistent symptoms can improve patient selection and post-procedure strategies. This study leverages data from the PRIME-MR registry to identify predictors of persistent symptom burden post M-TEER.
Methods
PRIME-MR is a retrospective, investigator-initiated registry including PMR patients who underwent M-TEER at 24 high-volume centers from 2009 to 2022. After excluding in complete cases, 888 patients were analyzed. Symptomatic status at discharge was classified as NYHA Class I/II or Class III/IV, with the latter indicating symptom persistance. The primary composite endpoint was all-cause mortality or rehospitalization.
Results
Among the 888 patients, 742 (83.6%) achieved NYHA Class I/II, while 146 (16.4%) remained in NYHA Class III/IV. Both groups were similar in age and sex distribution. However, those patients with persistent symptoms had higher initial NYHA classes (NYHA Class ≥III: 93.8% vs. 81.6%; p<0.001) and a shorter 6-minute walk distance (median 158 m [IQR 40, 270] vs. 252 m [137, 329]; p=0.006). Baseline MR severity (median EROA 0.4 cm² [0.3, 0.6]) did not differ ( p=0.81), but symptomatic patients had higher rates of mitral annular calcification (7.1% vs. 2.7%, p=0.023) and a smaller mitral valve orifice areas (4.0 cm² [3.1, 5.2] vs. 4.9 cm² [4.0, 5.8]; p=0.001). Moderate or greater residual MR at discharge was more frequent in symptomatic patients (56.6% vs. 28.9%; p<0.001) and linked to higher all-cause mortality or rehospitalization rates within 2 years (log-rank p<0.0001, Figure 1).
Conclusion
A distinct subset of patients undergoing M-TEER for primary MR does not experience symptomatic improvement at discharge, particularly those with mitral annular calcification and smaller mitral valve orifice areas. This subgroup was at significantly higher risk for mortality or rehospitalization. Tailored risk assessment and management strategies are crucial for improving outcomes in these high-risk patients.